



Vaccine Has 'Strong' Link With Bleeding Calf Syndrome
EUROPE – A highly fatal new born calf condition has had links with a Bovine Viral Diarrhoea (BVD) vaccine confirmed by a research consortium.Veterinary researchers from across Western Europe have released findings implicating the BVD vaccine PregSure as a factor in the rise in Bovine neonatal pancytopenia (BNP) or ‘bleeding calf syndrome’.
Report findings were based on a calf-focused analysis of risk factors that concentrated on the colostrum of vaccinated dams.
PregSure was withdrawn from the market in 2010 as a precaution to reports of internal and external haemorrhaging in calves, first surfacing in Germany in 2007.
Upon initial discovery, laboratories could not find a causative agent. The list of suspected BNP causes included poisoning, genetic disorders and reactions to drugs or vaccines.
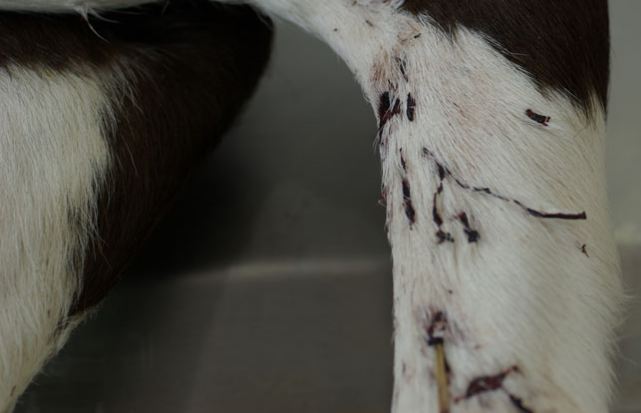
The consortium, led by Professor Dirk Pfeiffer, Royal Veterinary College, London, set out to analyse the link between dam vaccination with PregSure and BNP in youngstock.
It concluded that there was ‘strong’ evidence that the vaccine is a ‘major risk’ for BNP, but not the only risk.
The scope of the study neglected genotype of either calf or dam, which the team said could, along with other factors, explain why BNP develops in such a small proportion of vaccine-exposed calves.
In a press release, the Royal Veterinary College explained that sporadic reports of BNP across Western Europe could rely on calf or dam genotype being a factor.
Similarly, the study isolated a small number of calves (4.5 per cent) as having BNP for which no association with a PregSure vaccinated dam or the colostrum therein could be found.
However the RVC said: “If calves are only given colostrum from unvaccinated cows then it is highly unlikely they will develop BNP.”
Furthermore, the team found that older PregSure vaccinated cows were more likely to have a BNP calf than younger vaccinated cows.
A further finding was that colostrum sourced from unvaccinated cows had a diluting effect on the vaccinated milk and ‘reduced the odds’ of BNP.
Implicated vaccine manufacturer, Pfizer Animal Health (now Zoetis), withdrew sales of PregSure as a precaution in 2010, the year the study commenced.
In a statement, Zoetis welcomed the results and the report which, 'represents a very detailed epidemiological case control study investigating factors associated with the occurrence of Bovine Neonatal Pancytopaenia (BNP).'
They said the results were consistent with other smaller studies in Germany and the UK.
The statement added that BNP incidence has been low, despite widespread use of PregSure.
In withdrawing the vaccine from sales, Zoetis have largely mitigated the threat of BNP, according to the UK agricultural department (DEFRA).
Speaking in March last year, a DEFRA spokesperson said: “There is no risk to humans, as the age of the animal affected is 0 – 4 weeks, so they would not be entering the food chain. The age at which they are affected is very consistent and the disease has never been seen in older animals.”
The study, funded by Pfizer, recruited 405 BNP cases from 330 farms across Belgium, France and the Netherlands. Each holding had four control animals of similar age (1154 calves).
Other research projects by other research groups are on-going.
The RVC reported that BNP is now being linked with antibodies in calf bone marrow and blood cells.
The RVC said: “These groups have reported that BNP is associated with the presence of PregSure-induced maternal alloantibodies against the calf’s bone marrow and blood cells, but there is debate about the specific antigens involved.”
Michael Priestley
News Team - Editor
Mainly production and market stories on ruminants sector. Works closely with sustainability consultants at FAI Farms



